Research
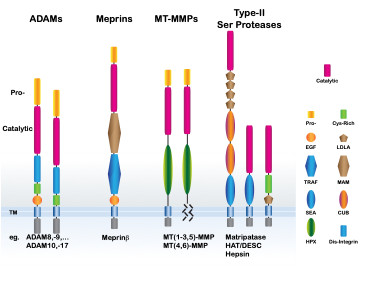
The human genome encodes more the 550 proteases with a growing number attributed to function in ectodomain shedding that control cell adhesion and signaling. Accordingly, these proteins are found in all aspects of human physiology and deregulated in disease pathogenesis. Many of these proteases, commonly called sheddases, are complex multi-domain proteins that use their ancillary and protease domains to process multiple different protein substrates (See Figure). Fittingly, many sheddases are strictly regulated by subcellular compartmentalization, zymogen maturation, temporal activation and biological inhibition. Yet a detailed molecular view in substrate selectivity and cellular regulation remains lacking. We aim to investigate the universal concepts that underpin the function of membrane-tethered proteases, such as the molecular basis for substrate selectivity and association with accessory proteins. We focus our efforts in biochemistry, cell biology and structural biology to study extracellular protein structure and function.